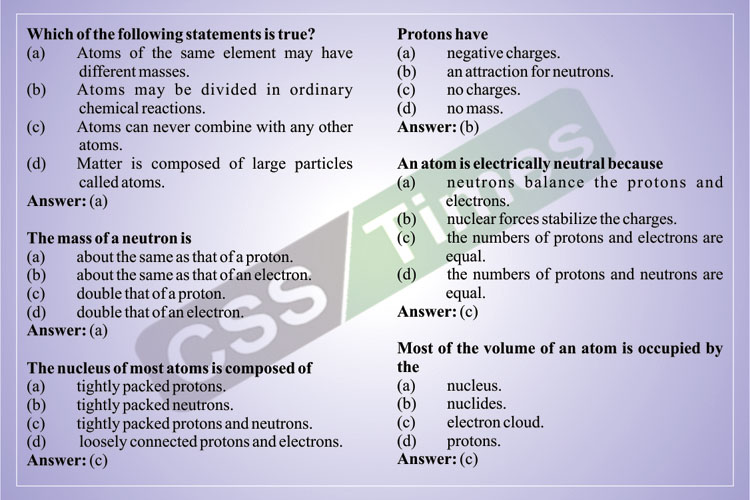
An Atom Is Electrically Neutral Because
- Atoms are electrically neutral because they have equal numbers of protons (positively charged) and electrons (negatively charged). If an atom gains or loses one or more electrons, it becomes an ion. If it gains one or more electrons, it now carries a net negative charge, and is thus 'anionic.'
- An atom is electrically neutral because it has equal quantities of positive and negative charges. The positive charge is due to the protons, which are.
The relative mass of an atom is almost the same as its nucleon number. The nucleon number is sometimes used as the approximate relative mass in calculations. Protons and neutrons are collectively called nucleons because protons and neutrons occupy the nucleus. In a neutral atom, number of electrons = number of protons.
By definition, an atom is electrically neutral (i.e. has the same number of protons as it does electrons, plus some number of neutrons depending on the isotope). If a species were charged, it is referred to as an ion (cation for positively charged and anion for negatively charged species), also by definition.
But this is probably not a very satisfying answer. (I personally find answers based on definitions pretty bland.) Perhaps an interesting follow up question is..
Is the universe electrically neutral?
For many instances in science, we deal with systems where charge neutrality is very important.
Perhaps a common example you might be familiar with is table salt, NaCl. Before forming salt, both sodium (Na) and chlorine (Cl) are electrically neutral atoms. Then chlorine nabs an electron from a sodium because it is more energetically favorable for it to have an additional electron. You then have a Na+ cation and Cl- anion that combine into NaCl due to electrostatic attraction. Overall, NaCl is a neutral system (table salt doesn't shock you when you eat it.. hopefully).
This property of electrical neutrality is also very important in the work that I do every day. I do computational research on crystals like NaCl where we calculate energies of a variety of sorts to understand the material. Part of calculating the total energy of a system for a crystal like NaCl involves the energy contribution that arises from Coulombic forces between every combination of Na+ and Cl- anion. This would mean figuring this out for something like 1023 ions (which is a lot). We do something a little more clever. NaCl is a crystal, which means it has a periodic (i.e. repeating) structure, so we only need to consider a unit cell, or small portion that can reproduce the entire crystal structure by translating it. But this means what we model is infinitely large materials. This is okay for bulk materials, since surface effects are small.
What is more worrying are those long range Coulombic forces. If we're not careful, we could end up with infinite energy! And that would be no good. This can be solved with a clever way of adding Coulombic forces (called Ewald summation) and a charge neutral unit cell.
An Atom Is Electrically Neutral Because *
But if many everyday things we are familiar with are electrically neutral, does this mean that the universe has to be electrically neutral?

Maybe.
It's actually still an open research question. What do you think would happen if the universe were just slightly positively charged overall? This is different from being ionized- that just means there are positively and negatively charged particles. But do these particles have to just balance each out? You can follow an interesting discussion here or a pretty recent article about how the universe could be slightly positively charged (the math gets a little hairy towards the end, but there luckily is more exposition overall).
Hope this helps!
Best,
An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons. The positive charges equal the negative charges, so the atom has no overall charge; it is electrically neutral. Most of an atom’s mass is in its nucleus; the mass of an electron is only 1/1836 the mass of the lightest nucleus, that of hydrogen. Although the nucleus is heavy, it is quite small compared with the overall size of an atom.
The radius of a typical atom is around 1 to 2.5 angstroms (Å), whereas the radius of a nucleus is about 10-5 Å. If an atom were enlarged to the size of the earth, its nucleus would be only 200 feet in diameter and could easily rest inside a small football stadium. The nucleus of an atom contains protons and neutrons. Protons and neutrons have nearly equal masses, but they differ in charge. A neutron has no charge, whereas a proton has a positive charge that exactly balances the negative charge on an electron. Table (PageIndex{1}) lists the charges of these three fundamental particles, and gives their masses expressed in atomic mass units.
| Particle | Charge | Mass (amu) |
|---|---|---|
| Electrons | -1 | 0.000549 |
| Protons | +1 | 1.00782 |
| Neutrons | 0 | 1.00867 |
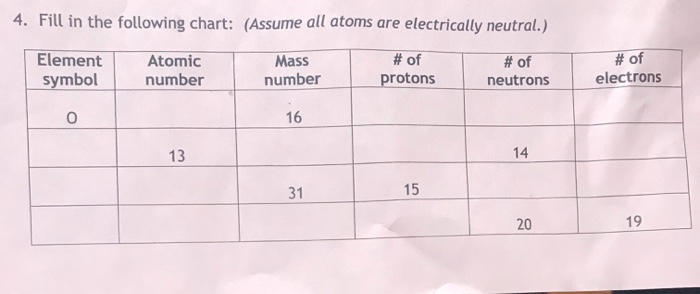
The atomic mass unit (amu) is defined as exactly one-twelfth the mass of a carbon atom that has six protons and six neutrons in its nucleus. With this scale, protons and neutrons have masses that are close to, but not precisely, 1 u each (there are 6.022 x 1023 u in 1 gram This number is known as Avogadro’s number, N, and one of the ways this number can be calculated is discussed below). The number of protons in the nucleus of an atom is known as the atomic number, Z. It is equal to the number of electrons around the nucleus, because an atom is electrically neutral. The mass number of an atom is equal to the total number of heavy particles: protons and neutrons.
When two atoms are close enough to combine chemically—to form chemical bonds with one another—each atom primarily “sees” the outermost electrons of the other atom. These outer electrons are therefore the most important factors in the chemical behavior of atoms. Neutrons in the nucleus have little effect on chemical behavior, and the protons are significant only because they determine how many electrons surround the nucleus in a neutral atom.
All atoms with the same atomic number behave in much the same way chemically, and are classified as the same chemical element. Each element has its own name and a one- or two-letter symbol (usually derived from the element’s English or Latin name). For example, the symbol for carbon is C, and the symbol for calcium is Ca. The symbol for sodium is Na-the first two letters of its Latin (and German) name, natrium,to distinguish it from nitrogen, N, and sulfur, S.
Example (PageIndex{1}): Bromine
What is the atomic symbol for bromine, and what is its atomic number? Zeppelin company logo. Why isn’t the symbol for bromine just the first letter of its name? What other element preempts the symbol B? (Refer to the periodic table)
Solution
Bromine’s atomic number is 35, and its symbol is Br; B is the symbol for boron
Contributors and Attributions
An Atom Is Electrically Neutral Because The
- Dickerson, Richard E. and Gray, Harry B. and Haight, Gilbert P (1979) Chemical principles.
